The Status of Zinc Pyrithione Regulation in Europe and Its Potential Ban
In recent years, the conversation around certain chemical compounds used in personal care products has intensified. Many shoppers are becoming more conscious of what goes into their everyday items, and the regulatory environment has been shifting in response to these demands. This raises an important question: are there specific substances currently facing restrictions that affect their availability in various markets, particularly in a well-known region?
Imagine a scenario where an effective solution for scalp conditions or acne is suddenly removed from shelves due to safety concerns or new regulations. This kind of situation sends ripples through consumers and industry professionals alike. Understanding the status of these compounds and the reasoning behind any imposed limitations can help buyers make informed choices.
In this article, we’ll dive into the current landscape of regulations regarding a particular antifungal agent. We’ll explore the reasons behind its scrutiny, the perspectives of health authorities, and what this means for consumers seeking effective treatments. With a focus on safety and efficacy, let’s unpack this unfolding narrative together.
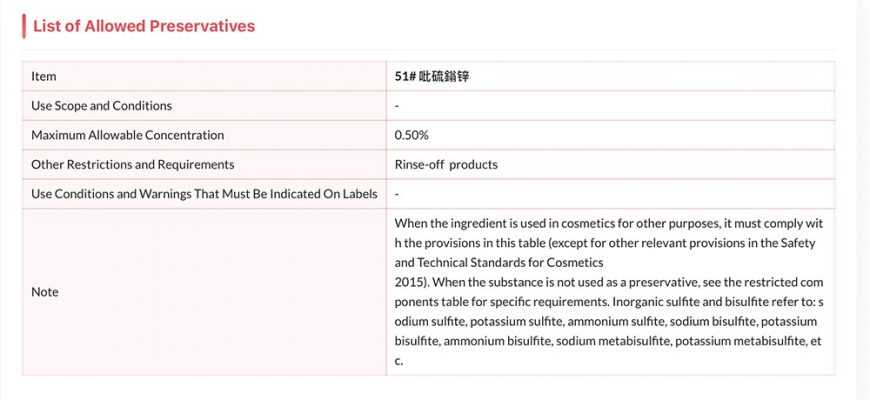
Regulatory Status of Zinc Pyrithione
The discussion surrounding the regulatory framework for certain substances often leads to confusion among consumers and manufacturers. Understanding the rules and guidelines is crucial for those involved in the production and use of various chemical agents, particularly in personal care and household products.
In recent years, authorities have been closely examining the safety profiles of specific compounds used for their antimicrobial and anti-fungal properties. This scrutiny aims to ensure that these agents pose minimal risk to human health and the environment. As part of ongoing evaluations, some ingredients have been placed under stricter controls, limiting their use in various applications.
Currently, there are specific regulations and recommendations issued by competency agencies that impact the formulation and distribution of these agents within certain markets. Manufacturers must stay informed of these legal updates to comply and continue their operations. This situation has prompted many companies to reformulate their products to align with the latest safety standards.
It’s essential for consumers to be aware of these dynamics as they make choices about the products they prefer. Understanding the regulatory landscape helps in making informed decisions and ensures that individuals are using safe and compliant products in their daily lives.
Health Concerns and Safety Assessments
In the realm of personal care and dermatological treatments, there tends to be a spotlight on certain substances due to their potential impact on health. Concerns often arise from scientific evaluations, leading to discussions about their safety and the risks they may pose to users. When it comes to topical agents, the conversation amplifies as individuals seek effective solutions while remaining vigilant about potential adverse effects.
Regulators and health organizations conduct thorough assessments to understand these compounds better. This analysis typically examines the risk of skin irritation, potential allergic reactions, and any long-term implications of regular usage. Findings from these assessments guide public opinion and influence manufacturing practices, ensuring consumer safety remains a priority.
In addition to regulatory oversight, ongoing research plays a crucial role in addressing any emerging concerns. Studies often delve into the mode of action and the interaction of these substances with human biology. The goal is to strike a balance between efficacy and safety, allowing individuals to make informed choices based on credible information.
Consumer vigilance is equally important in this conversation. Individuals are encouraged to stay informed about the products they use, considering not just effectiveness but also the safety profile of each ingredient. Engaging with reputable sources and staying updated on scientific findings can empower users in making choices that prioritize their health.
Impact on Consumer Products in Europe
The recent regulatory changes have significantly influenced various products available to consumers. As manufacturers adapt to these new rules, many formulations are undergoing transformations to ensure compliance, which can lead to alterations in product efficacy and performance.
Shifts in ingredient availability force brands to seek alternatives. This not only impacts the quality of items, but also consumer choices. Some individuals may find that their preferred goods are reformulated, which can be a point of frustration for loyal users who have specific expectations.
Additionally, the modification of certain substances leads to a change in market dynamics. Manufacturers must navigate the delicate balance of maintaining effectiveness while adhering to safety standards. This challenge prompts many companies to invest in research and development, potentially paving the way for innovative solutions that could benefit consumers in the long run.
Ultimately, the implications of these regulatory decisions extend beyond just product formulation. They influence consumer behavior and may encourage individuals to seek out alternatives, reshaping the landscape of personal care and household items available on store shelves.